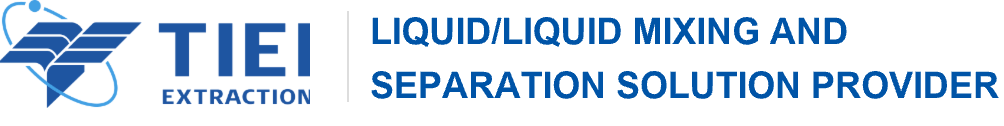Extraction is a technique that separates compounds based on solubility. Depending on the phases involved, extractions are either liquid-solid or liquid-liquid. If you have ever brewed a cup of tea or boiled bones for soup, you have performed a liquid-solid extraction. Compounds that are soluble in hot water (the liquid) are extracted from tea leaves or bones (the solid). Flavoring extracts are also made by liquid-solid extraction.
In organic laboratory, liquid-liquid extraction is most commonly used. Liquid-liquid extraction requires two immiscible liquids known as the organic phase and the aqueous phase. The aqueous phase is water-based and can be an acidic,basic, neutral, or a saturated salt solution. The organic phase is an organic solvent, usually diethyl ether or dichloromethane, which has minimal solubility in water. For instance, ethanol would be a poor extraction solvent because it forms a solution with water. Organic extraction solvents do not mix with water, they form distinct layers, much like oil and water. Compounds can be separated based on which liquid they are more soluble in.
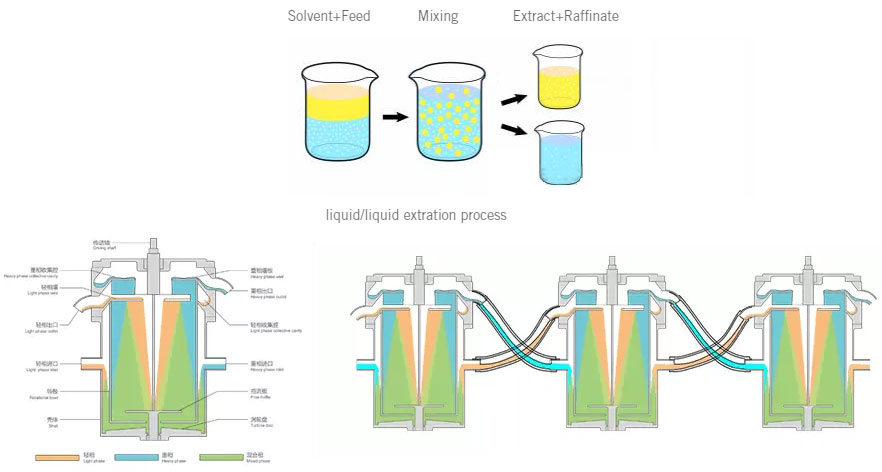
While limited mass transfer can be completed in a single, batch equilibrium contact of the two phases, one of the primary advantages of liquid-liquid extraction processes is the ability to operate in a continuous, multistage countercurrent mode. This allows for very high separation factors while operating at high processing rates. Countercurrent operation is achieved by repeating single-stage contacts, with the aqueous and organic streams moving in opposite directions.

Zhengzhou Tiei Extraction Technology Co.,Ltd designs, manufactures CWL-M centrifugal extractor for the liquid/liquid separation. CWL650-M successfully applied in lactic acid purify from Fermentation , at Asia biggest factory.